insights into the packing switching of the epha2 transmembrane domain by molecular dynamic simulations
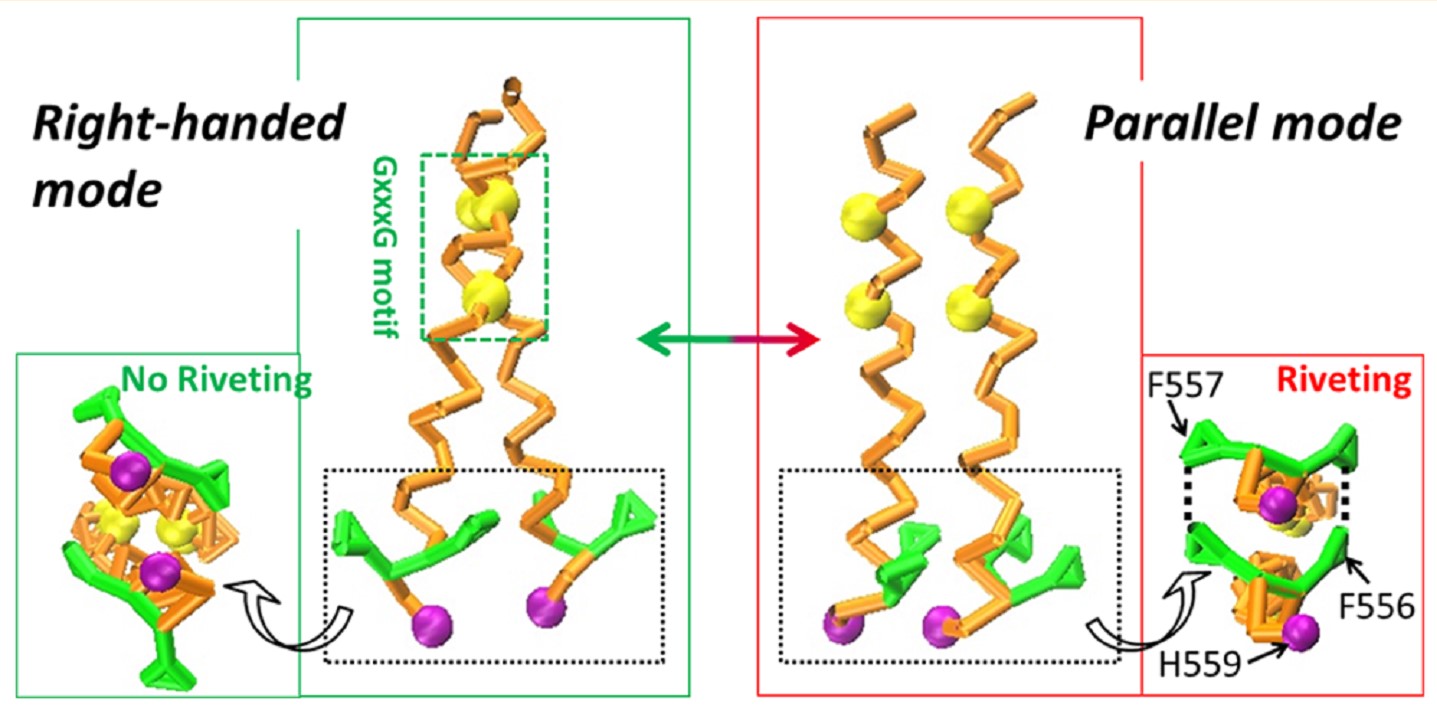
abstract: receptor tyrosine kinases play an important role in mediating cell migration and adhesion associated with various biology processes. with a single-span transmembrane domain (tmd), the activities of the receptors are regulated by the definite packing configurations of the tmds. for the epha2 receptor, increasing studies have been conducted to investigate the packing domains that induce its switching tmd dimerization. however, the inherent transformation mechanisms including the interrelations among the involved packing domains remain unclear. herein, we applied multiple simulation methods to explore the underlying packing mechanisms within the epha2 tmd dimer. our results demonstrated that the g 540 xxxg 544 contributed to the formation of the right-handed configuration while the heptad repeat l 535 xxxg 539 xxa 542 xxxv 546 xxl 549 xxxg 553 motif together with the ffxh 559 region mediated the parallel mode. furthermore, the ff 557 residues packing mutually as rigid riveting structures were found comparable to the heptad repeat motif in maintaining the parallel configuration. in addition, the h 559 residue associated definitely with the lower bilayer leaflet, which was proved to stabilize the parallel mode significantly. the simulations provide a full range of insights into the essential packing motifs or residues involved in the switching tmd dimer configurations, which can enrich our comprehension toward the epha2 receptor
